Rigorous maths is not essential for the sake of this course, so that will only be added later if required.
Diffraction
Geometry
| Symbol | Meaning |
|---|---|
| \(\mathbf{r}\) | Position |
| \(\mathbf{s_0}\) | Unit vector of incident beam |
| \(\mathbf{s}\) | Unit vector of diffracted beam |
| \(\mathbf{S=(s-s_0)}/\lambda\) | Scattering vector |
With such definitions, the phase difference is \(\mathbf{r\cdot S}\).
Diffracting from electron density
For the most part, only elastic scattering is considered. The amplitude of the diffracted beam remains the same, but there is a phase shift.
We define a transmission function, which depends on the electron density, \[ \psi(\mathbf{r})=\exp(i\sigma \rho(\mathbf{r})), \] so that the total diffracted amplitude is \[ \Psi=\int\psi(\mathbf{r})\exp(2\pi i \mathbf{r\cdot S})\, dV_r. \] The kinematic approximation is a Taylor expansion of the transmission function, \[ \psi (\mathbf{r})\approx 1+i\sigma\rho(\mathbf{r}). \] Then, ignore the constant 1, and define the very convenient structure factor as \[\begin{align} F(\mathbf{S})&=\frac{\Psi}{i\sigma}\\ &=\int\rho(\mathbf{r})\exp(2\pi i\mathbf{r\cdot S}). \end{align}\] Now the electron density and the diffracted amplitude are related by a Fourier transform - if we know the full information of \(F\), we know the electron density.
The phase problem
In reality, only the diffracted intensity is measurable, hence the size of \(F\), but not the phase. To measure the phase, one would need an aerial comparable to the wavelength, but the reason why we are doing X-ray at the first place is that it’s comparable to atoms.
Therefore, the main task in structure determination is to deduce the phase angle of each structure factor.
Patterson Function
If we don’t know it, just ignore it.
Since only the intensity can be measured, we try to deduce as much information as possible from the intensity alone. The Patterson function is the inverse FT of \(|F|^2\) (which is basically the intensity). \[\begin{align} P(\mathbf{u})&=\mathcal{F}^{-1}[FF^*]\\ &=\mathcal{F}^{-1}[F]*\mathcal{F}^{-1}[F^*]\\ &=\rho(\mathbf{r})*\rho(-\mathbf{r})\\ &=\int\rho(\mathbf{r})\rho(\mathbf{u-r})\, dV_r \end{align}\] The second term is valid, because \(\rho\) is real, so it’s FT has conjugate symmetry.
The Patterson function can be interpreted as the overlap between two copies of electron densities, if one is translated by \(\mathbf{u}\).
- always symmetrical about origin
- maximum at \(\mathbf{u=0}\)
- creates a peak when \(\mathbf{u}\) is an interatomic vector
- weight of peak is product of no. of electrons
- there are \(N^2-N\) non-origin peaks
- peaks twice as broad
Gas phase
The radial Patterson function is extremely useful when dealing with gas phase diffractions, because
- Intermolecular vectors are averaged out as a weak background
- Intramolecular vectors are spherically averaged, hence only depend on the distance between pairs of atoms.
Single crystal
Summing up contributions of all cells, the intensity is given by \[ I(\mathbf{S})=|F_\mathrm{cell}|^2\frac{\sin^2(\pi(N\mathbf{a\cdot S}))}{\sin^2(\pi(\mathbf{a\cdot S}))}. \]
- Maxima at \(\mathbf{S\cdot a}\) is integer;
- N-2 subsidiary maxima in between;
- For very large N, this becomes an array of \(\delta\), referred to as reciprocal lattice.
Independent Atom Model
Chemists generally are only interested in the positions and types of atoms. The useful assumptions for this purpose are:
- The electron density is not continuous, but gathered into isolated, individual atoms at fixed sites.
- Atoms can be considered spherical.
- All electrons are localized within atoms - neglect electrons in bonds.
Now the electron density in the crystal is a convolution between \(\rho_\mathrm{atom}\) and a delta function of their positions in the lattice.
Consequences for structure factors
Hence, \[\begin{align} F(\mathbf{S})&=\sum_\mathrm{atom\ types} \mathcal{F}[\rho_\mathrm{atom}]\times\mathcal{F}[\mathrm{lattice}]\\ &=\sum_\mathrm{all\ atoms} f_n\exp[2\pi i \mathbf{r \cdot S}]. \end{align}\] In other words, \[ \sum f_n \exp [2\pi i \mathbf{r \cdot S}] =\int \rho(\mathbf{r})\exp [2\pi i \mathbf{r \cdot S}]\, dV_r. \]
- When \(\mathbf{S}=0\), \(F(0)=\int \rho(\mathbf{r})\, dV_r\) is the number of electrons.
The scattering factor \(f_n\) are usually fitted as a function of \(\sin\theta/\lambda\).
- Larger radius, or the consideration of atomic displacements, both reduces the peak width of \(f_n\).
Symmetry in Crystals
Symmetry elements
Crystals have inherent translational symmetry.
Other symmetry elements, and its result in the reciprocal space are as follows. (Detailed derivations pp. 30-37)
| Symmetry | Reciprocal space | Comment |
|---|---|---|
| Inversion centre * | Different probability distribution of intensities; possible for very weak F(hkl) | Reciprocal lattice is always centrosymmetric (Friedel’s law) |
| Mirror plane | A mirror plane \(\perp \mathbf{b}\) gives a mirror plane \(\perp \mathbf{b^*}\) | |
| Rotation axis | Produces the corresponding rotation axis. | If 2-fold rotation along one axis, mirror perpendicular to that axis, then that axis is perpendicular to both other axes. |
| Screw axis | Along \(\mathbf{b}\) produces a corresponding rotation axis; (0k0) absent for odd k. | |
| Glide plane | \(\mathbf{c}\) glide perpendicular to \(\mathbf{b}\) produces a mirror plane \(\perp \mathbf{b^*}\); (h0l) absent for odd l. | |
| Unit-cell centering | P - no absence; C - absent for odd (h+k); I absent for odd (h+k+l); F absent - unless all even or all odd. |
Space groups
The name consists of two parts
- Centering - P, C, I or F
- Symmetry elements along each axis.
Only need to know \(P2_1\), \(P2_1/c\), \(P2_1 2_1 2_1\). The first two groups are abbreviated by ignoring the ‘1’ (stands for no symmetry) for a and c.
General equivalent positions are positions related by the symmetry elements of the space group. There are (m+1) of them.
For \(P2_1/c\), the conventions are:
- Screw axes at (0, y, 1/4)
- c-glide at (x, 1/4, z)
Symmetry in Patterson function
The Patterson peaks between two GEPs are guaranteed to exist. These vectors and produced by substracting the GEP coordinates. If it is a heavy atom, then the peaks are expected to be strong, and can be used to straight away to work out there positions.
Note some pairs of GEPs will produce the same vector, hence systematic doubling the Patterson intensities of these peaks.
Heavy Atom method
By decomposing the vector \[ F(hkl)=F_\mathrm{heavy}(hkl)+F_\mathrm{rest}(hkl), \] it can be approximated that, given \(|F_\mathrm{heavy}|\gg|F_\mathrm{rest}|\), the overall phase \(\Phi(hkl)\approx\Phi_\mathrm{heavy}(hkl)\).
Sequence
- Determine the positions of heavy atoms from Patterson
- Evaluate \(F_\mathrm{heavy}\)
- Take \(\Phi(hkl)=\Phi_\mathrm{heavy}(hkl)\)
- Generate \(\rho_\mathrm{trial}=\mathcal{F}^{-1}(|F|\exp{(i\Phi)})\)
- Try to deduce any new identities of atoms
- Regenerate \(F_\mathrm{heavy}\)
- Repeat
Special case of centrosymmetric structures
The phase can be either 0 or \(\pi\), so if the heavy atoms are really dominant, the approximate phase is exactly correct. The true \(\rho\) can be generated in one inverse FT.
Limitations
- Heavy atoms not dominant enough (hence the approximation of phase is bad)
- Heavy atoms on SEPs
Phase Determining methods
If the heavy atoms are not dominant enough, then the approximation of the phase is bad; however, geometrically, if the known \(F_\mathrm{heavy}\) can be altered in a known way, than \(F\) can be solved in a triangle.
Isomorphous Replacement
If an isomorphous crystal can be produced by replacing the known heavy elements, then
- new \(F_\mathrm{heavy}'\) is also known, with the phases being equal \(\Phi_\mathrm{heavy}=\Phi_\mathrm{heavy}'\)
- for the rest of atoms, \(F_\mathrm{rest}\) is unchanged
- the size and phase of the difference in the structure factor, \(\delta F\) is known.
Now we know \(|F|\), \(|F'|\) and \(\delta F\), a triangle can be constructed to solve for \(F\), up to a mirror image.
A multiple isomorphous replacement can be carried out to confirm which triangle the true \(F\) is in - out of the four triangles generated in two sets of experiments, only one edge is shared.
Anomalous scattering
It is sometimes too hard to make isomorphous crystals, but the known \(F_\mathrm{heavy}\) can also be changed by changing the scattering power of these elements.
When the X-ray is shorter in wavelength than the absorption edge of an element, significant absorption takes place. The transmission function is now \[ \psi(xyz)=\exp(i \sigma\rho(xyz))\exp(-\eta(xyz)). \] As a result, the diffracted amplitude now has an imaginary component \[ F(hkl)=\mathcal{F}[\rho(xyz)]+\frac{i}{\sigma}\mathcal{F}[\eta(xyz)]. \] Therefore, the difference \(\delta F\) always has a phase of \(\Phi_\mathrm{heavy}+\frac{\pi}{2}\), and now \(|F(hkl)|\neq|F(\bar{h}\bar{k}\bar{l})|\). It is possible in this case to deduced the absolute structure (handedness of the structure).
Otherwise, the process of determining the phase is similar to above.
Note that a cyclotron can be used to generate variable X-ray wavelengths.
Direct Methods
Idea
When no heavy atom is present, no positions can be deduced from the Patterson function, and hence the previous methods can not be applied.
The fact that the electron density \(\rho(xyz)\) is a positive quantity imposes restrictions on phases. It can therefore help us rule out certain combinations of phases, and work towards the correct structure. It is useful because the process can be automated.
(All examples concern special cases of real \(F(hkl)\), i.e. choices between 0 and \(\pi\) only.)
Fixing the origin
Phases change when the origin changes, since \[ F(\mathbf{S})=\sum_n f_n \exp(2\pi i[\mathbf{S}\cdot (\mathbf{r_n}-\mathbf{r_O})]). \] Before starting to work on choices, the origin must be fixed.
In a centrosymmetric group, inversion about each SEP generates specific phases \(0\) or \(\pi\) for different even/odd combinations of \(hkl\) values. (e.g. \(P2_1 /c\) on pp.59-60) By arbitrarily choosing 3 phases (usually 100, 010, 001), it is sufficient to fix the origin on a specific SEP.
Expanding by symmetry
Known group gives known GEPs; with the known 3 arbitrary choices, we can deduce the phases of all \(F(hkl)\) which are related to these 3 points by symmetry. With \(m\) symmetry elements in a group, we now know the phases of \(3m\) structure factors.
Projected structures
Purpose
The larger \(|F(hkl)|\), the stronger it contributes to the Fourier summation of the electron density, the more confidence we have when assigning its phase.
However, in a triple summation over \(hkl\) with no heavy atoms involved, no term can be particularly dominating. By projecting onto 2D or 1D, we only deal with 2D or 1D summations, where strong \(F(hkl)\) can be more dominating. Projections can be done by, e.g. onto body diagonal in a centrosymmetric group, \[ \rho(xxx)=F(000)+2\sum_h S(hhh)|F(hhh)|\cos(2\pi(3hx)). \]
New problems
Many overlaps will be generated, and hence there will be very little near-zero areas left. Now, even a few (weak) phases are wrongly assigned, no significant negative areas will be generated.
This can be solved, by trying to further reduce the IAM into point atom models. Then, the fuzziness of atoms will be removed, and overlaps will be reduced.
In the reciprocal space, this means that the atomic scattering factors should not drop off with \(\theta\), but remains quite constant. This can be achieved by using unitary scattering factor, \[ n_n=\frac{f_n}{\sum_i f_i}. \]
The \(f_n\) for all atoms drop off in a similar fassion, so at any particular \(\theta\), the ratio between \(f_n\) for different atoms is roughly constant.
Now unitary structure factors are \[ U(\mathbf{S})=\sum_n n_n \exp(2\pi i(\mathbf{S}\cdot\mathbf{r}_n)). \] It is helpful to further normalize \(U\) to eliminate the effect of systematic intensity changes, to give normalized structure factor \[ E(\mathbf{S})=\frac{U(\mathbf{S})}{\sum_n n_n}\\ |E(\mathbf{S})|^2=\frac{|F(\mathbf{S})|^2}{\epsilon {\sum_n f_n}}. \] where \(\epsilon\) is the systematic intensity change caused by symmetry.
Pair relationships
In 1D projection, e.g. if \(|E(h)|\approx |E(2h)|\), and are strong compared to others. By trying all four combinations of signs, \(S(2h)\) must be +1 to avoid two troughs coinciding.
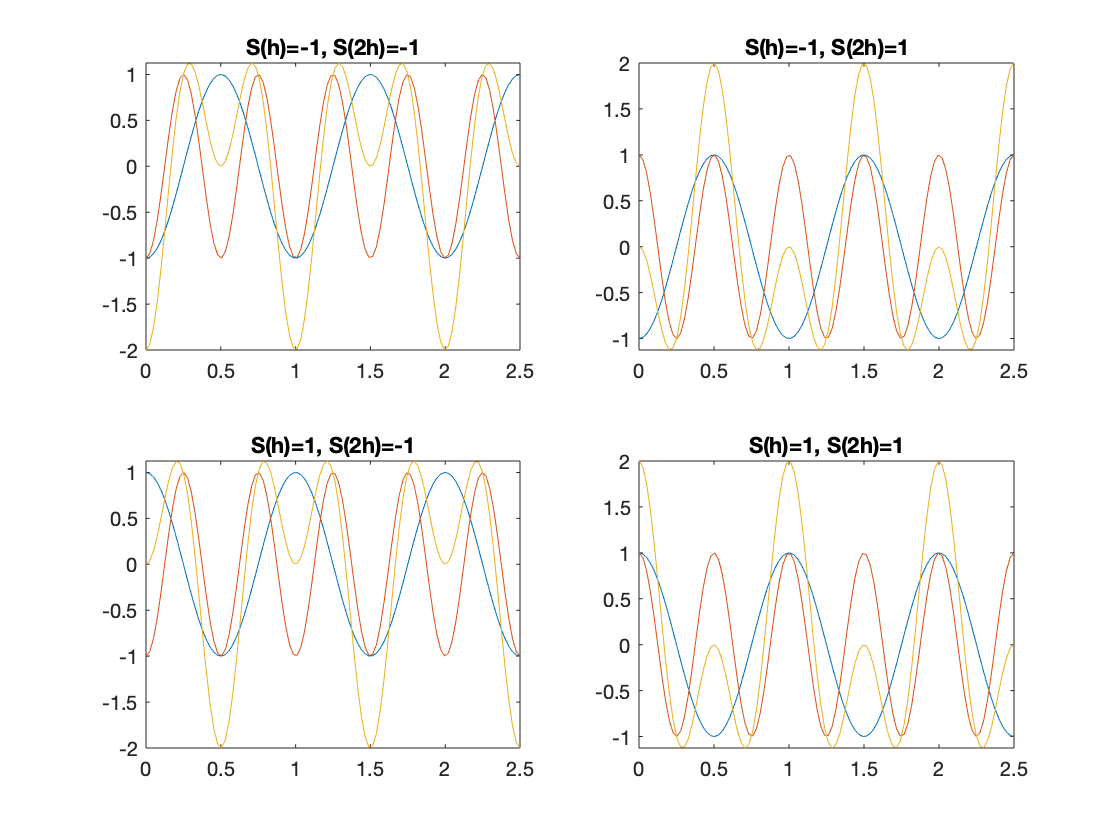
Triplet relationships
Similar idea, in 2D projections, between \(S(\mathbf{h})\), \(S(\mathbf{h'})\) and \(S(\mathbf{h-h'})\).
By trying all combinations of phases, and to ensure that any two coinciding troughs are cancelled by a peak, it can be concluded that \[ S(\mathbf{h})S(\mathbf{h'})S(\mathbf{h-h'})\approx+1, \] where \(\approx\) says ‘probably equals to’.
Not suitable
When
- Heavy atoms are present - truncation error can produce significant negative regions next to a big peak.
- Macromolecular structure - unlikely to see particularly strong peaks.
The Rest
Dual-space methods
An iterative method, by forcing any negative \(\rho\) value to be positive.
Structure refinement
All the above methods are approximate. To get a more accurate structure, we would want to minimize any difference between observed and calculated \(|F|\).
Difference density
- any +ve peaks are missing atoms. - excellent way to find H atoms
- +ve/-ve peaks next to an atom position - incorrect position
- hollow peaks at atom position - displacement parameter makes the atom too dense.
Always use chemical sense!